A strong 13C chemical shift signature provides the coordination mode of histidines in zinc-binding proteins.
Résumé
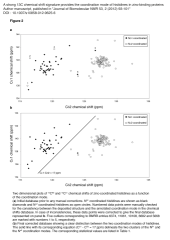
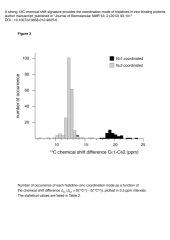
Zinc is the second most abundant metal ion incorporated in proteins, and is in many cases a crucial component of protein three-dimensional structures. Zinc ions are frequently coordinated by cysteine and histidine residues. Whereas cysteines bind to zinc via their unique Sγ atom, histidines can coordinate zinc with two different coordination modes, either Nδ1 or Nε2 is coordinating the zinc ion. The determination of this coordination mode is crucial for the accurate structure determination of a histidine-containing zinc-binding site by NMR. NMR chemical shifts contain a vast amount of information on local electronic and structural environments and surprisingly their utilization for the determination of the coordination mode of zinc-ligated histidines has been limited so far to 15N nuclei. In the present report, we observed that the 13C chemical shifts of aromatic carbons in zinc-ligated histidines represent a reliable signature of their coordination mode. Using a statistical analysis of 13C chemical shifts, we show that 13Cδ2 chemical shift is sensitive to the histidine coordination mode and that the chemical shift difference δ{13Cε1} - δ{13Cδ2} provides a reference-independent marker of this coordination mode. The present approach allows the direct determination of the coordination mode of zinc-ligated histidines even with non-isotopically enriched protein samples and without any prior structural information.
Domaines
Biochimie, Biologie Moléculaire
Fichier principal
 Barraud_JBiomolNMR_2012.pdf (689.73 Ko)
Télécharger le fichier
Barraud_JBiomolNMR_2012.pdf (689.73 Ko)
Télécharger le fichier
 Barraud_Fig1.png (148.12 Ko)
Télécharger le fichier
Barraud_Fig1.png (148.12 Ko)
Télécharger le fichier
 Barraud_Fig2.png (271.69 Ko)
Télécharger le fichier
Barraud_Fig2.png (271.69 Ko)
Télécharger le fichier
 Barraud_Fig3.png (113.6 Ko)
Télécharger le fichier
Barraud_Fig3.png (113.6 Ko)
Télécharger le fichier
 Barraud_Fig4.png (221.36 Ko)
Télécharger le fichier
Barraud_JBiomolNMR_2012-Poster.pdf (813.51 Ko)
Télécharger le fichier
Barraud_Fig4.png (221.36 Ko)
Télécharger le fichier
Barraud_JBiomolNMR_2012-Poster.pdf (813.51 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Autre